Cis-trans preference is expressed as a one-fold cosine function in torsion term. To properly describe cis-trans preference of N-methyl acetamide, the k2 value(spring constant of one-fold cosine function. f(theta)= k1*(1+cos(2*theta-180)) + k2*(1+cos(1*theta))) seems to be around 1.5 kcal/mol.
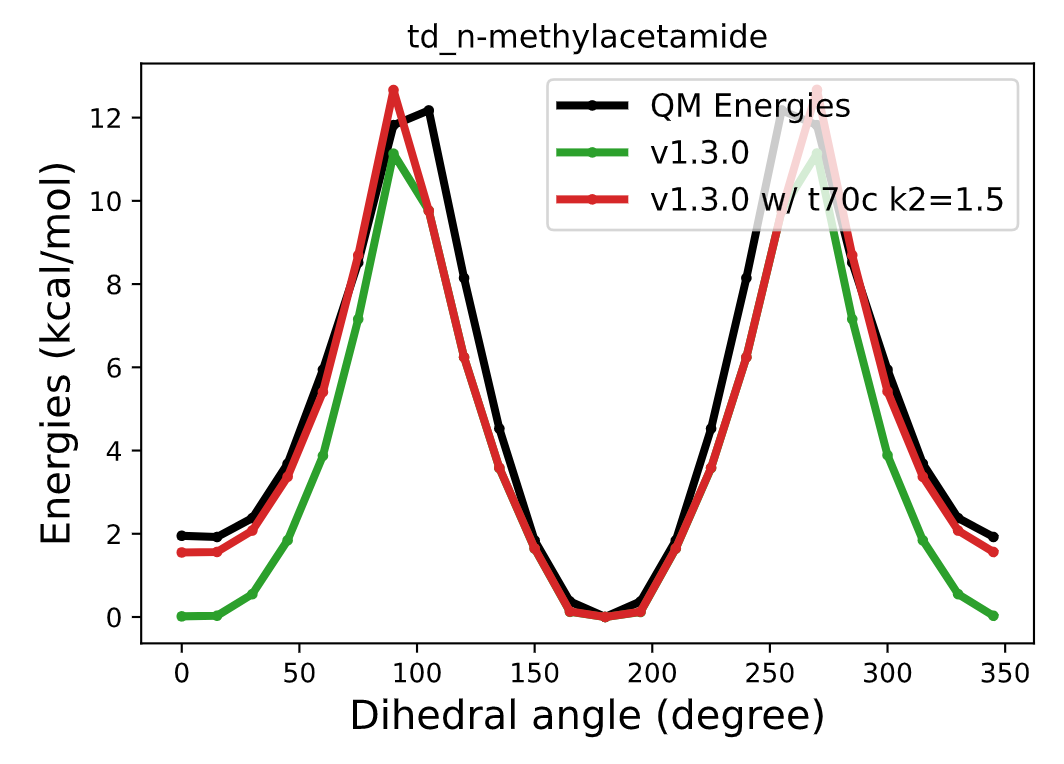
Torsional energy profiles of N-methyl acetamide
Initial guess for the k2 was set to 2.0kcal/mol, which seems a pretty reasonable value, but it decreases during the fitting to QM amide torsional energy profiles. (in fitting v1.3.0)
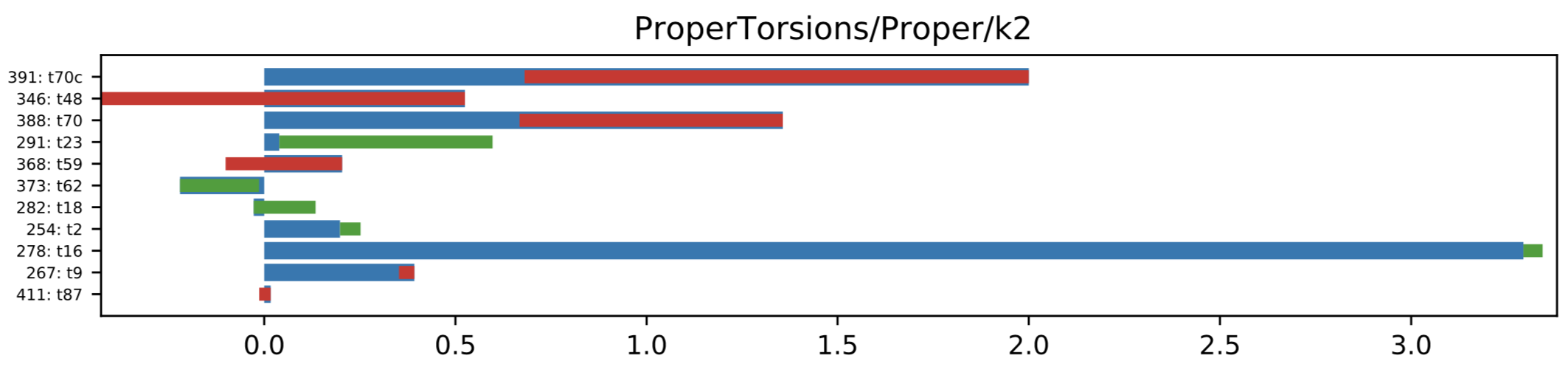
Torsion k2 parameter changes during the optimization
To figure out the origin that decreases the k value of one-fold term, gradient contribution at the initial guess has been plotted.
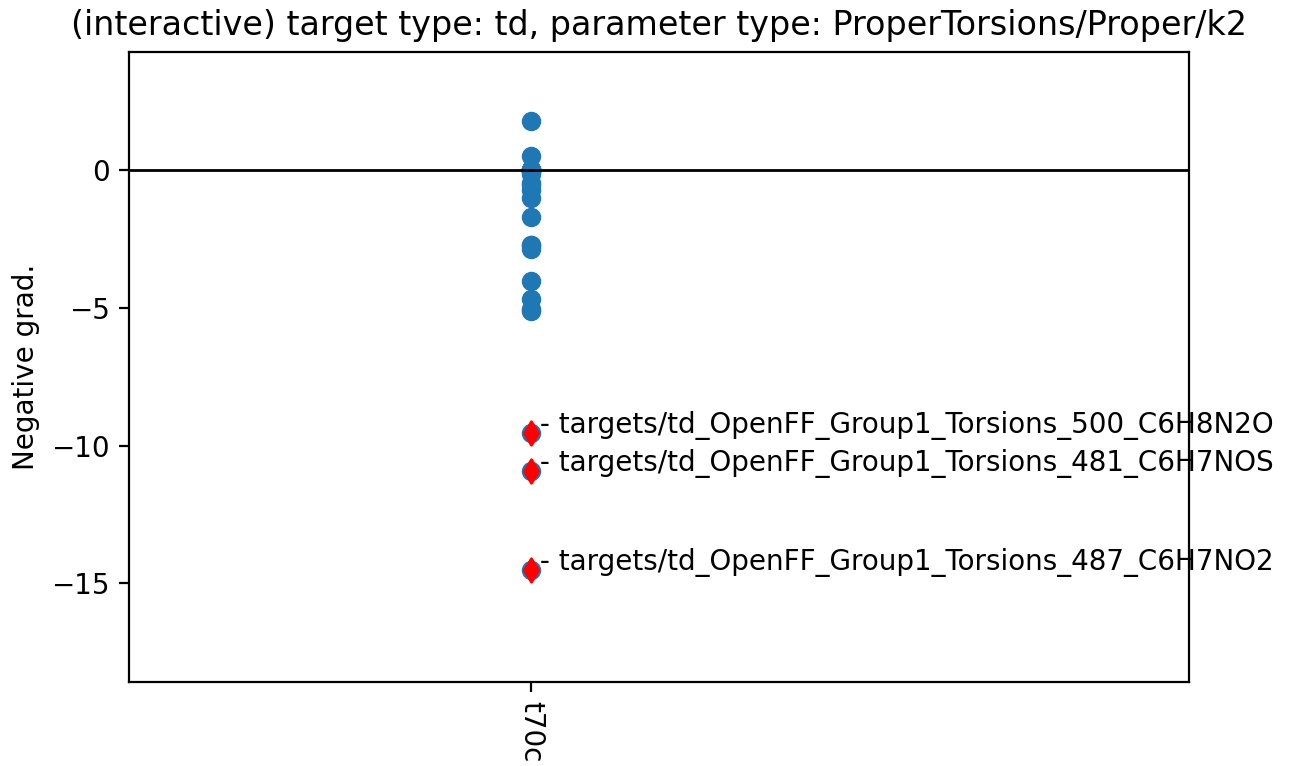
Negative gradient w.r.t the spring constant of one-fold cosine of t70c
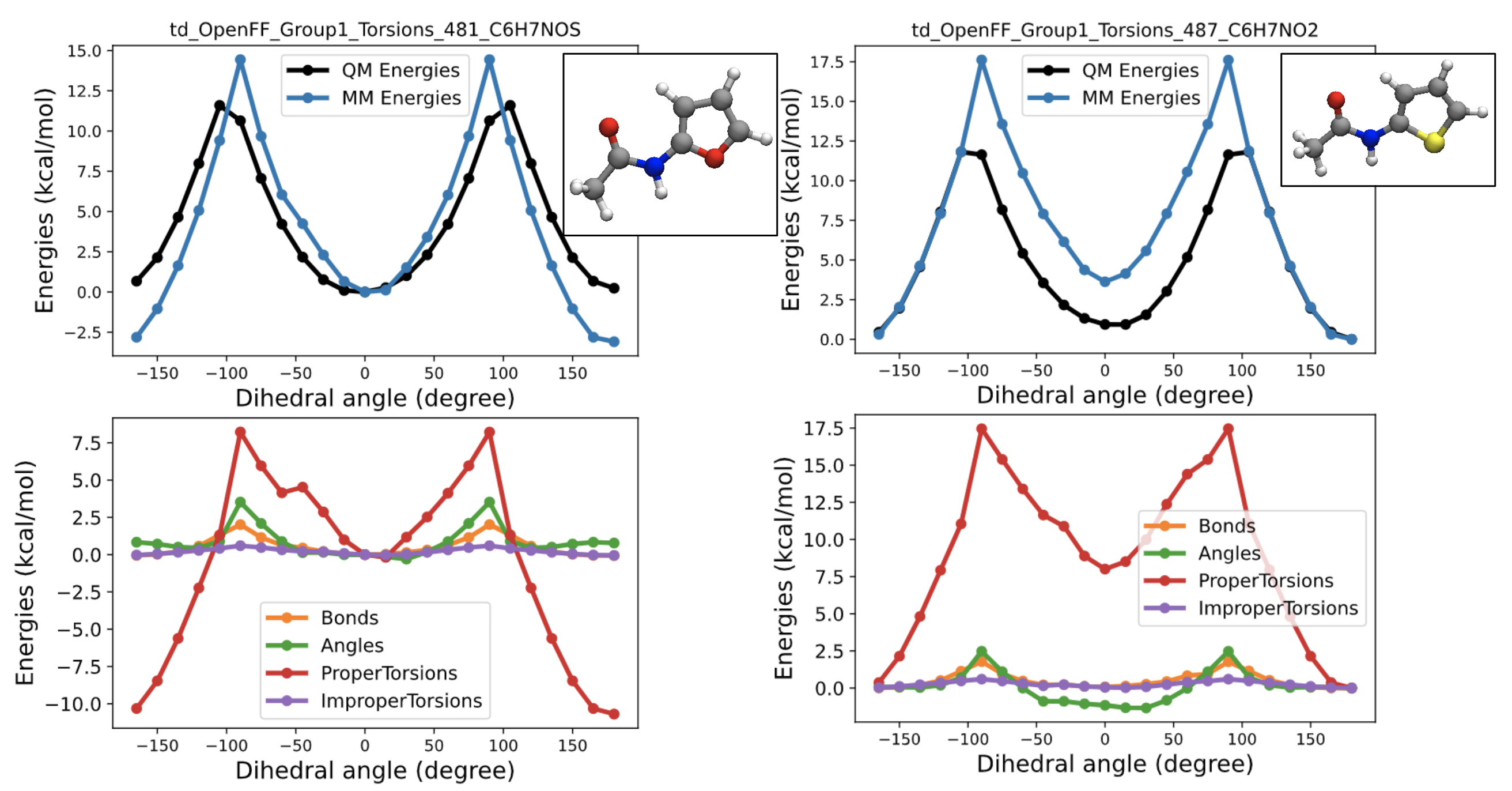
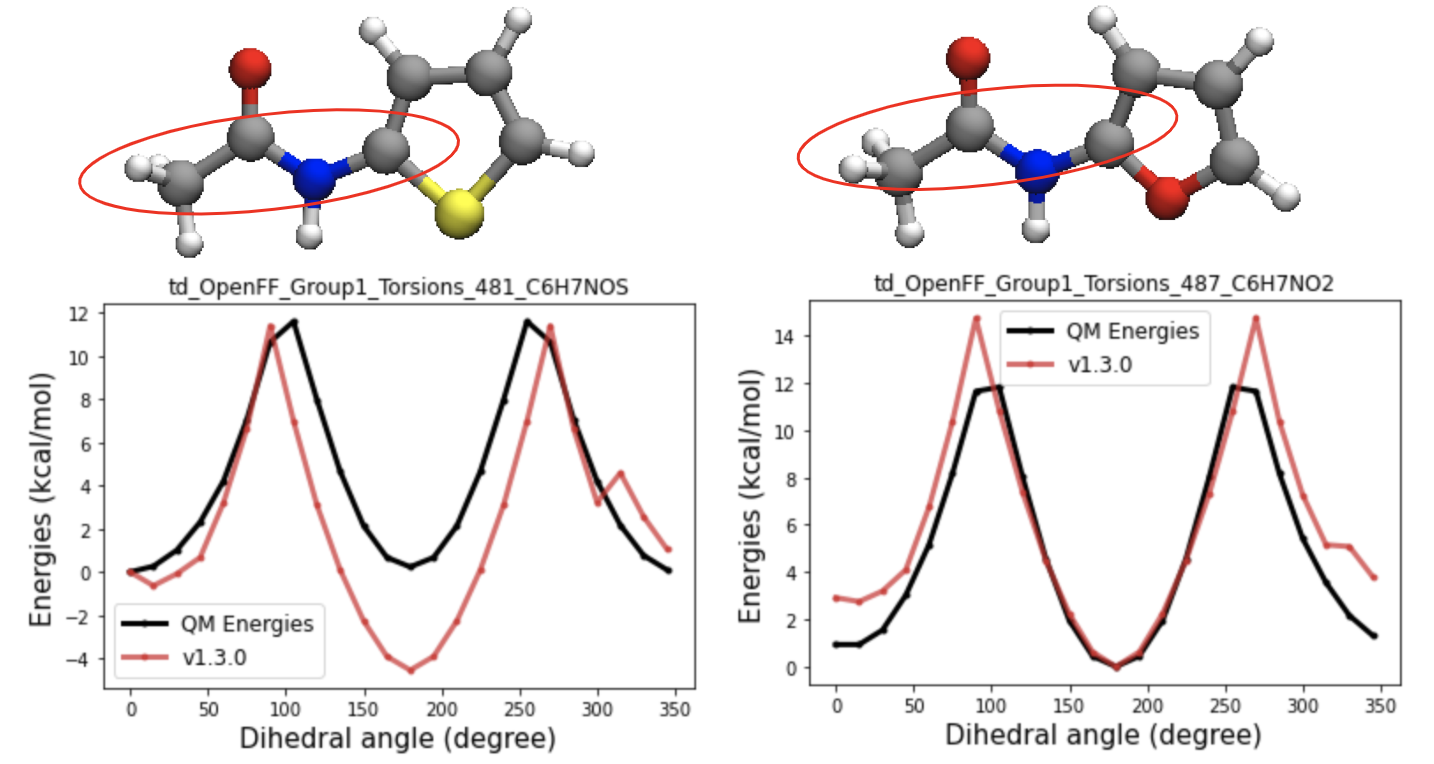
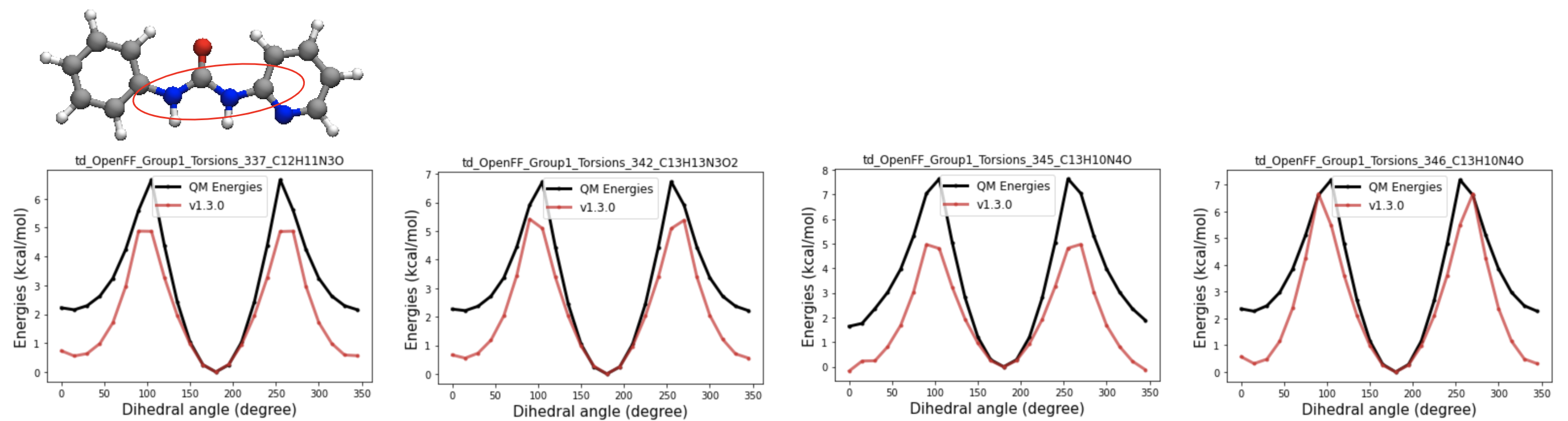
2D images and energy decoupling plots of the targets with the biggest gradient contribution to the spring constant of one-fold cosine of t70c
QM energy profiles of the targets with the most negative gradient contribution w.r.t the t70c k2 value show the energy gap between cis and trans conformers are much smaller(close to zero) than other amides. Therefore, separating those from t70c might be able to allow t70c to have large enough k2 value, so that it can keep the t70c term from losing its cis trans preference. So i came up with the new child parameter of t70, t70e and ran test fitting to see the impact of the additional parameters.
List of child parameters of t70 in the current force field(v1.3.0)
t70 [#1:1]-[#7X3:2]-[#6X3:3]=[#8,#16,#7:4]
t70b [*:1]-[#7X3:2]-!@[#6X3:3](=[#8,#16,#7:4])-[#6,#1]
t70c [#1:1]-[#7X3:2]-!@[#6X3:3](=[#8,#16,#7:4])-[#6,#1]
t70d [*:1]-[#7X3:2]-!@[#6X3:3](=[#8,#16,#7:4])-[#7X3]
(1) RC2
Goal: test separation of torsions having sp2 carbon attached to the amide nitrogen from t70c + consideration of cis/trans preference of amide with sp3 N attached to the amide carbon
parameters added:
t70e [#1:1]-[#7X3:2](-[#6X3])-!@[#6X3:3](=[#8,#16,#7:4])-[#6,#1] (between t70c and t70d)
t70f [#1:1]-[#7X3:2]-!@[#6X3:3](=[#8,#16,#7:4])-[#7X3] (after t70d)
resulting parameters:
| k1*(1+cos(2*theta-180)) + k2*(1+cos(1*theta)) | |
|---|---|---|
| [#1:1]-[#7X3:2]-!@[#6X3:3](=[#8,#16,#7:4])-[#6,#1] | k1=2.49e+00, k2=1.54e+00 |
| [#1:1]-[#7X3:2](-[#6X3])-!@[#6X3:3](=[#8,#16,#7:4])-[#6,#1] | k1=2.47e+00, k2=9.12e-01 |
| [*:1]-[#7X3:2]-!@[#6X3:3](=[#8,#16,#7:4])-[#7X3] | k1=6.08e-01 |
| [#1:1]-[#7X3:2]-!@[#6X3:3](=[#8,#16,#7:4])-[#7X3] | k1=6.98e-01 , k2=8.60e-01 |
While the k2 values show good separation of t70e from t70c, it failed to improve the torsional energy profiles of the torsions assigned to t70e.
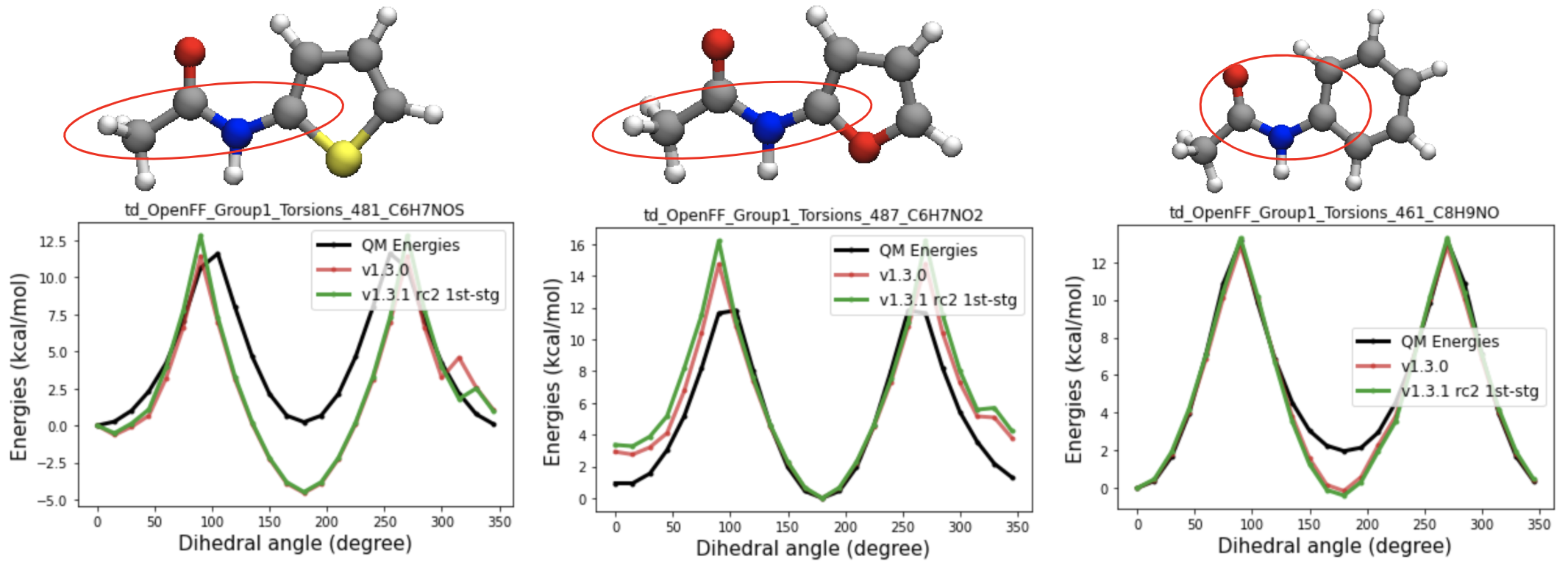
A closer look at the QM torsional energy profiles t70e being fitted, shows that depending on the functional groups attached to the amide nitrogen, they get different cis-trans preferences. So for RC6 and RC7, I defined more specific SMIRKS for t70e(in-ring sp2 carbon having a single bond with either oxygen or sulfur(RC6) or sp2 carbon in 5-membered ring(RC7)) and fitted the parameters.
(2) RC6
param modification:
t70e [#1:1]-[#7X3:2](-[#6X3](-@[#8,#16]))-!@[#6X3:3](=[#8,#16,#7:4])-[#6,#1] (between t70c and t70d)
t70f [#1:1]-[#7X3:2]-!@[#6X3:3](=[#8,#16,#7:4])-[#7X3] (after t70d)
resulting parameters:
| k1*(1+cos(2*theta-180)) + k2*(1+cos(1*theta)) | |
|---|---|---|
| [#1:1]-[#7X3:2]-!@[#6X3:3](=[#8,#16,#7:4])-[#6,#1] | k1=2.47e+00, k2=1.55e+00 |
| [#1:1]-[#7X3:2](-[#6X3](-@[#8,#16]))-!@[#6X3:3](=[#8,#16,#7:4])-[#6,#1] | k1=2.45e+00, k2=-6.24e-01 |
| [*:1]-[#7X3:2]-!@[#6X3:3](=[#8,#16,#7:4])-[#7X3] | k1=5.81e-01 |
| [#1:1]-[#7X3:2]-!@[#6X3:3](=[#8,#16,#7:4])-[#7X3] | k1=1.42e+00 , k2=1.10e-01 |
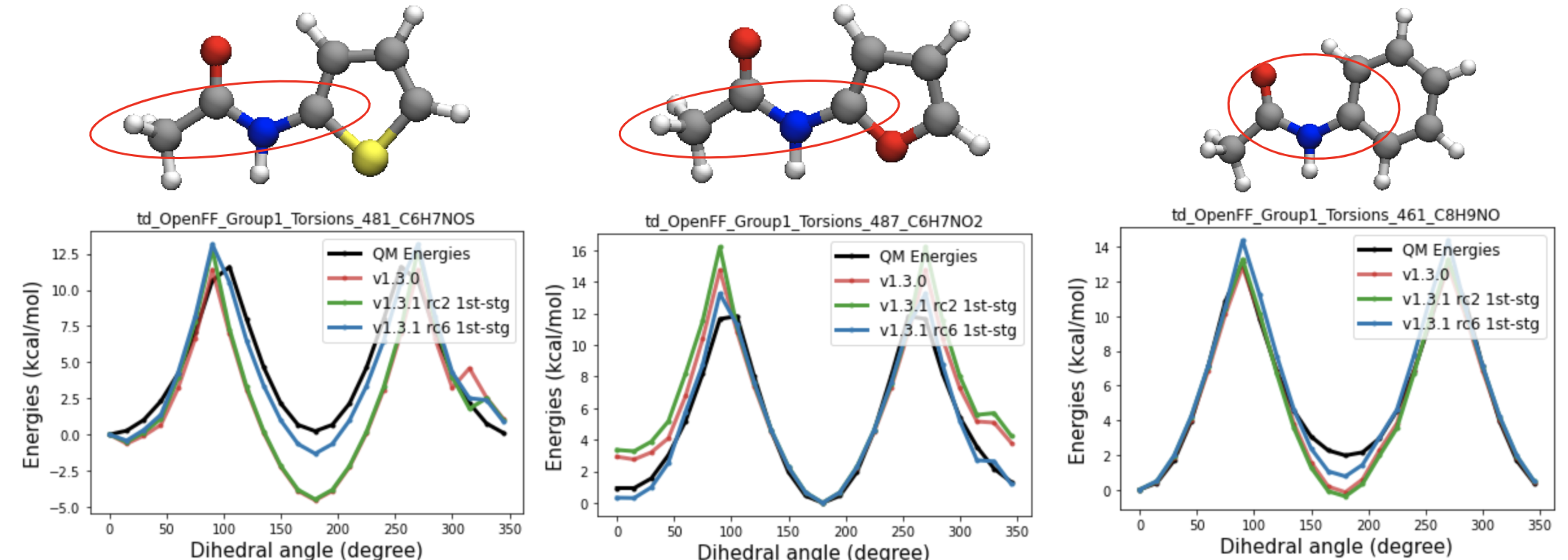
Separation of the first two rotations(no significant cis-trans preference) and the last molecule into different parameter shows improvement in cis-trans preference predictions.
(3) RC7
param modification:
t70e [#1:1]-[#7X3:2](-@[#6X3;r5])-!@[#6X3:3](=[#8,#16,#7:4])-[#6,#1] (between t70c and t70d)
t70f [#1:1]-[#7X3:2]-!@[#6X3:3](=[#8,#16,#7:4])-[#7X3] (after t70d)
similar result with RC6.
There are some local environments on amides, which affect cis-trans preference of the amides. One example is N-thiophen-2-ylacetamide, whose amide nitrogen has a thienyl group. The energy gaps between cis and trans conformer of the molecule is almost zero.
Preliminary fittings show that separation of the case where local environment introduces low energy gap between cis and trans conformers can fix the reproduction of cis-trans preference of N-methylacetamide.
More molecules with different functional groups on the amide nitrogen side are needed to determine (1) whether the new torsion parameter is needed(whether the electrostatic interaction plays a major role in the different cis-trans preferences between amides or the local environment really affects on the torsional energy) (2) the most reasonable SMIRKS for the new parameter.